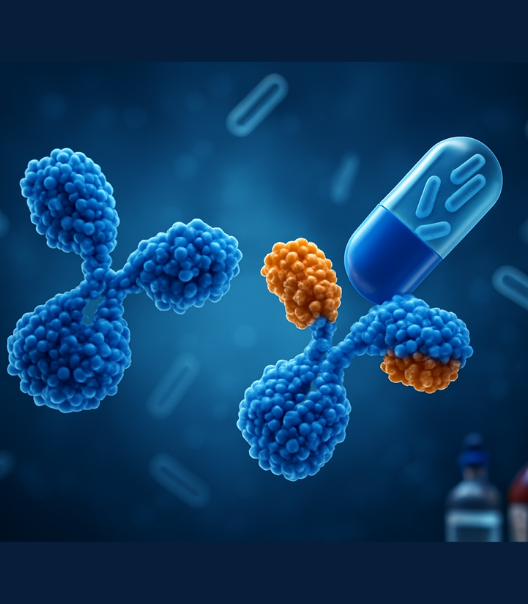
An All-in-One Solution for
Affordable Biologics
Transforming Research Into Real-World Impact
Glogene is revolutionizing biologics development by blending cutting-edge science with a commitment to
global health. We specialize in the development and manufacturing of monoclonal antibodies, fusion proteins,
and complex biologics, helping our partners accelerate
their journey from discovery to clinic with speed,
quality, and confidence.
We are redefining biologics development through our CHO platform and Glo-Fusion™ technologies. As a trusted partner to biotech innovators and global pharmaceutical companies, we provide customized, end-to-end solutions that accelerate the journey from discovery to commercial launch.
Our Promise
Innovation-driven solutions - Advancing biologics with cutting-edge science and technology
Seamless Development & Manufacturing - Ensuring smooth integration from discovery to delivery
Affordable, High-Quality, Compliant - Delivering trusted and accessible therapeutics through operational excellence
Our Focus Areas
Cell Line Development - High-yield, stable cell lines powered by our CHO platform
Process Development - Scalable upstream and downstream solutions through our GloFusion™ platform
Analytical & Characterization - Comprehensive analytical and bioanalytical services for quality and compliance
GMP Manufacturing - From clinic to commercial supply
Pre Clinical & Clinical Support - From early studies to clinical trials with regulatory-ready solutions

Core Values
Innovation
At Glogene, innovation is not just about breakthroughs - Transforming ResearchInto Real-World Impact

Commitment to Affordable Access
We are dedicated to making advanced biologics more affordable and accessible

Integrity, Quality & Compliance
We are guided by three core values: Integrity, Quality, and Compliance

Respect
Creating an environment where every person is heard, valued, and empowered to contribute to the organization's goal

Our leadership team brings together over 100 years of combined scientific and strategic expertise,
spanning biosimilar and monoclonal antibody development, regulatory strategy, and global
biopharmaceutical commercialization. With this depth of experience, we guide Glogene’s vision to
accelerate innovation, ensure compliance,
and deliver sustainable impact on a global scale.
Glogene Technologies
Cell Line Development
Efficient. Scalable. Reliable.
The CHO platform delivers high-yield, stable clones with unmatched speed and productivity. Built on a CHO-GS platform, engineered for fast growth, strong secretion, and optimal glycosylation, it accelerates cell line development while ensuring monoclonality and long-term stability.
Glo-Fusion™ - Upstream Process Development
Next-Generation Perfusion (NGP) Technology
Glo-Fusion™ integrates advanced NGP strategies to deliver intensified,high-cell-density, and high-productivity upstream processes with superior speed,scalability, and robustness. With unmatched productivity, Glo-Fusion™ enables reliable and cost-effective manufacturing to meet global demand.
Our Services
Your partner from Gene to GMP-accelerating development of monoclonal antibodies, fusion proteins, and complex biologics with robust & compliant processes.

Cell Line Development
Advanced cell line development with our CHO platform
Upstream Process Development
Robust upstream process development for optimized biologic yield

Downstream Process Development
Efficient downstream process development for high-purity biologics

Process Characterization and Validation
Comprehensive process characterization and validation for reliable outcomes

Product Characterization
In-depth product characterization for regulatory compliance and safety

Drug Product Development
Integrated strategies for safe and effective drug product development
Manufacturing
State-of-the-art manufacturing solutions to accelerate market readiness
Our Work Flow
Clone to Clinic in Just few Months with Glo-Technologies
Our CHO and Glo-Fusion™ platforms enable rapid biologics development,
compressing timelines
from cell line cloning to clinical candidate ready for trials within few months.
Cell Line Development
From vector design to monoclonality, built for consistent performance and commercial success
Process Optimization
Optimization of upstream and downstream processes for higher yield and quality.
Analytical
Robust analytical characterization to confirm consistency, quality, compliance, and performance across biologics
Clinical Studies
End-to-end clinical study support, from trial material manufacturing to regulatory documentation aligned with global compliance
Regulatory Approval
Comprehensive regulatory approval support-streamlining submissions,ensuring compliance, and accelerating global market entry